Introduction
Copper (Latin cuprum) is a chemical element with the element symbol Cu and atomic number 29. It is a transition metal, in the periodic table it is in the 4th period and the 1st subgroup (according to new counting group 11) or copper group. The Latin name cuprum is derived from (aes) cyprium "ore from the Greek island of Cyprus", where copper was mined in ancient times.
As a relatively soft metal, copper is easy to shape and tough. As an excellent conductor of heat and electricity, it has many uses. It also belongs to the group of coinage metals.
As an important technology or functional metal, copper belongs to the group of semi-precious metals.
Mining
Copper ores are often contain sulphur. Sulphuric acid can be released from mining dumps through weathering processes and washed into the surrounding area by rainwater. The groundwater is because of that in dangerous.
Processing
In the next step , sulphur is going to be seperated from copper. It ist possible that because of that the smelling of SO2 ist very strong und it can cause irritation of the mucous membranes.
Acid rain
Even far-off regions can be affected, as pollutants can be transported by the wind. In this way soil organisms are disturbed and plant communities are damaged.
Example
Copper is often used in cabels and pans. It is a good conductor of electricity. 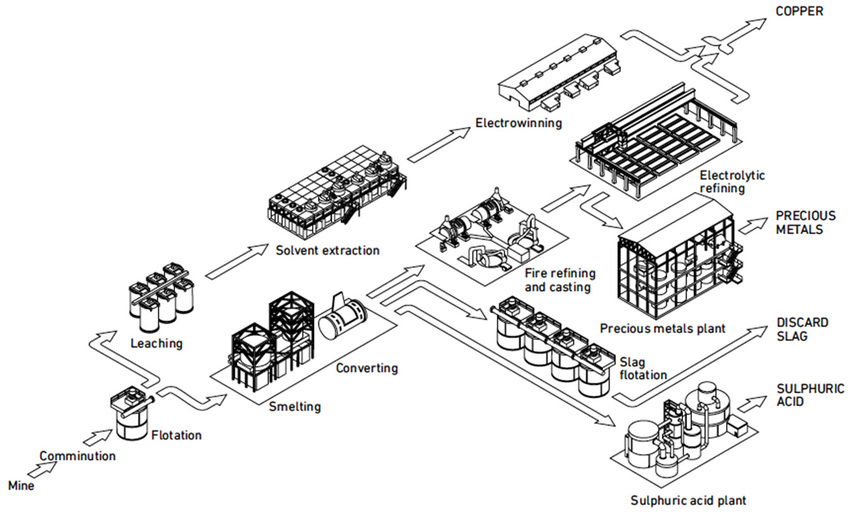
https://dontwastemy.energy/2017/01/20/copper/