Teaser
Hydrogen (Wasserstoff) was discovered in 1766 by Henry Cavendish, an English scientist, when he made base metals react with acids (Säuren). He called the element "phlogiston". In 1783, A. L. de Lavoisier succeeded in synthesizing the gases hydrogen and oxygen to water. Lavoisier then named hydrogen after two Greek (griechisch) terms: hydro (water) and gennao (I generate) as "hydrogene" – “I generate water”. In 1814, the element symbol "H" was introduced by Berzelius, a Swedish chemist. But how has this element changed over the years?
100 years ago
Elemental (molecular) hydrogen serves as a starting material to produce ammonia, hydrochloric acid (Salzsäure), methanol, aniline, and many other substances. It is used as a welding gas (Schweissgas). In the past, hydrogen was also used as a filler for balloons. However, in the Lakehurst disaster, a zeppelin named Hindenburg exploded in New York in 1937. This airship contained 200,000 m3 of hydrogen. A fire caused the entire airship to explode. 35 people lost their lives.

Picture of the Hindenburg disaster
In 1965/66 hydrogen fuel cells were used in space missions such as Gemini and Apollo. Just 6 years before the new millennium (Jahrhundert) began, the first hydrogen fueled car was introduced. And just 5 years later the first hydrogen fueling stations (Wasserstofftankstellen) in Hamburg and München were built.
Today
Hydrogen is the smallest and most common element in the universe. The colourless and odourless (geruchslos) gas appears almost only in bonded form, for example hydrogen is mainly found in H2O, that means it comes mainly together with oxygen in form of water.
Because of the different ways in which hydrogen can be produced, hydrogen is divided into different colours. For us, the green hydrogen is the most important because it is the only right option when it comes to sustainably reducing CO2 emissions.
How is hydrogen produces and where is it used?
- There is more than one process to produce hydrogen. Green hydrogen is produced by the electrolysis (Elektrolyse) of water using electricity from renewable sources (erneuerbare Quellen): wind or photovoltaic.
- By applying an electrical voltage, water is broken down into its components hydrogen (H2) and oxygen (O) in the electrolyser (Elektrolyseur). The protons form hydrogen molecules at the negative pole, which rise and are captured.
- The so-called blue hydrogen is obtained from petroleum gas (Erdgas) (CH4). There are also several processes for this, for example pyrolysis (Pyrolyse) or steam reforming (Dampfreformierung).
- Steam reforming uses water vapour (Wasserdampf) to separate the hydrogen contained in the petroleum gas from the carbon and obtaining pure hydrogen. The carbon monoxide produced during steam reforming is converted into carbon dioxide (CO2).
- In methan pyrolysis (Methanpyrolyse), petroleum gas is broken down into its carbon and hydrogen components in a high-temperature reactor.
- Carbon dioxide, more precisely carbon, can be stored in deep geological structures or used in industrial processes for example. This avoids the release into the atmosphere.
- The hydrogen can be transported directly via the existing gas infrastructure to the users and can be consumed there, for example in fuel cell heating systems (Brennstoffzellen-Heizungen), in vehicles or in industry.
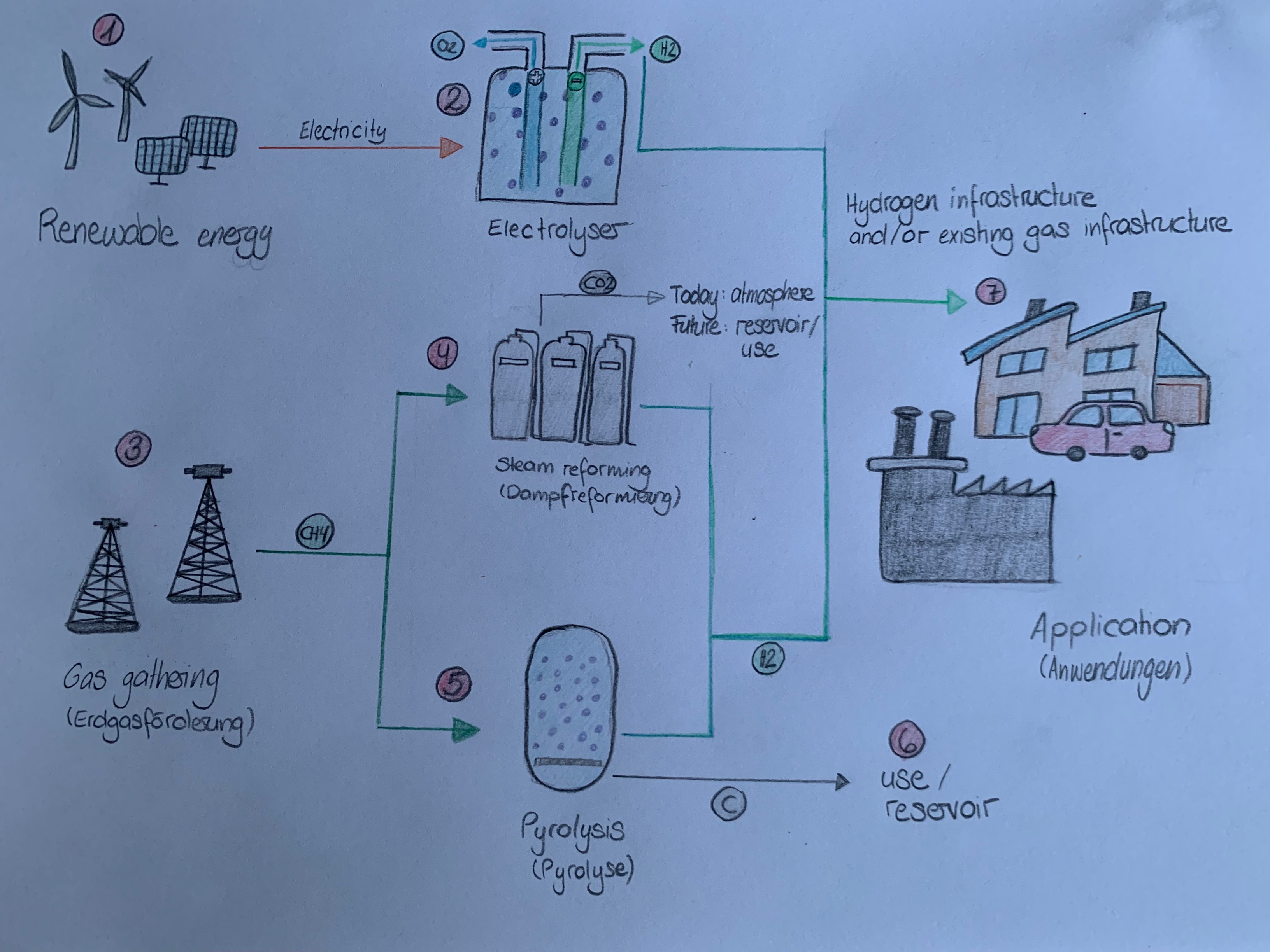 Hydrogen production (selfmade drawing)
Hydrogen production (selfmade drawing)
In 100 years
What is currently still an obstacle (Hindernis) to the full use of hydrogen in the future are the high production costs. Renewable energies should also be further expanded. Currently, green hydrogen costs more than twice as much to produce as other types/colours of hydrogen. But chemists are confident, that this should change in the next few years. They mean, that the more people use hydrogen, the more the prices will go down.
Another problem is the storage of hydrogen. This is supposed to be a lot more difficult than for fossil fuels because it has a very low density (geringe Dichte). H2 is the lightest gas in the universe. It is also highly explosive. The gas would have to be pressed into special containers under high pressure or it would have to be stores as a liquid at -253 degrees celsius.
Green hydrogen does n ot generally solve our energy problem, but it can make an important contribution (Beitrag) to several sectors such as heavy goods transport (Schwerlastverkehr) or industry.
Summary
Anabel & Sandrine
PDF-File:
IDAF Hydrogen
Text sources:
lernhelfer.de
ffe.de
bdew.de
tuev-nord.de
dw.com
Picture sources:
Hindenburg disaster: wikipedia.org
Hydrogen production: bdew.de